São Paulo, Brazil – The Butantan Institute, linked to the state of São Paulo’s Health Department, on December 12 submitted a request for the use of Brazil’s first chikungunya vaccine to Anvisa, the country’s national health monitoring agency.
The vaccine, developed in partnership with the Franco-Austrian pharmaceutical company Valneva, has proven to be safe and effective in two phase 3 clinical trials, with the second trial coordinated by the Butantan Institute involving Brazilian volunteer teenagers.
The study showed that the vaccine induced the production of neutralizing antibodies in 98.8% of the volunteers. According to the Ministry of Health, until early September, Brazil had 82 deaths from chikungunya and 143,000 cases in 2023.
José Moreira, medical director of Clinical Development at Butantan, said that chikungunya is a quite peculiar disease, as 30% to 35% of infected people develop chronic joint pain. This situation can negatively impact people’s quality of life.
“Therefore, having a vaccine with the potential to protect against the disease represents an instrument that could be used as a public health measure, both during outbreaks or even incorporating this vaccine into the national immunization calendar,” Moreira told Brazil Reports.
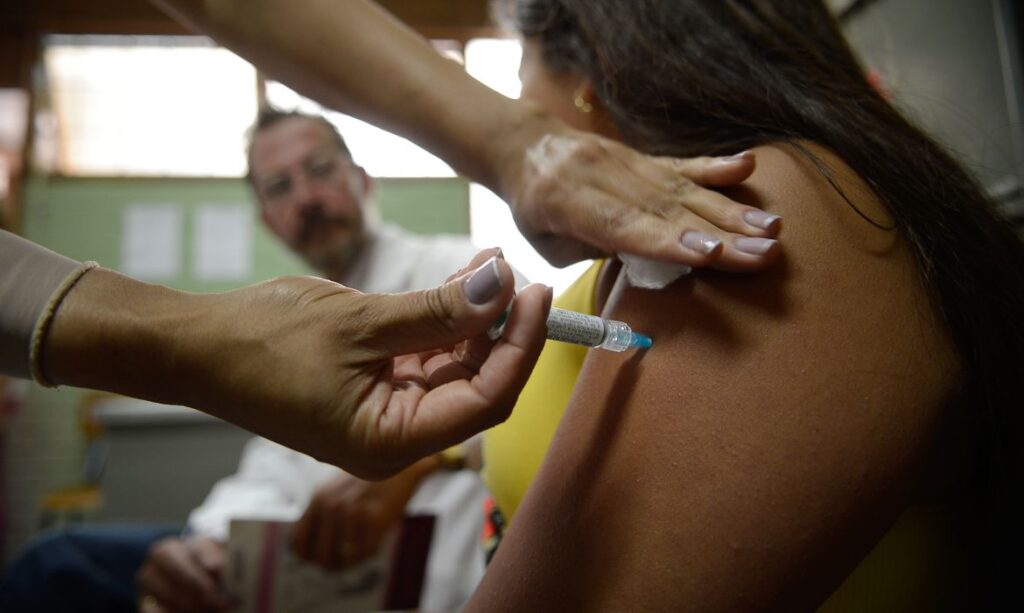
About the vaccine
The research and production of the chikungunya live-attenuated virus vaccine is the result of a technology transfer agreement signed by Butantan with Valneva in 2020. The phase 3 clinical trials of the vaccine, conducted in Brazil and the United States, showed that it has a good safety profile and high immunogenicity.
Among the 4,000 adult and elderly volunteers aged 18 to 65 evaluated in the US, 98.9% produced antibodies, with levels remaining robust for at least six months of follow-up. The results of the US study were published in June.
In Brazil, the study was conducted in 750 volunteers aged 12 to 17 living across 10 cities in areas where chikungunya is endemic. Among people with no previous contact with the chikungunya virus, 98.8% presented antibodies; for the group with a history of previous infection, the antibody positivity was 100%.
“These data confirm that the administration of a single dose of the vaccine induces robust protection against chikungunya and a well-tolerated safety profile in individuals living in an endemic region for the disease,” said Moreira.
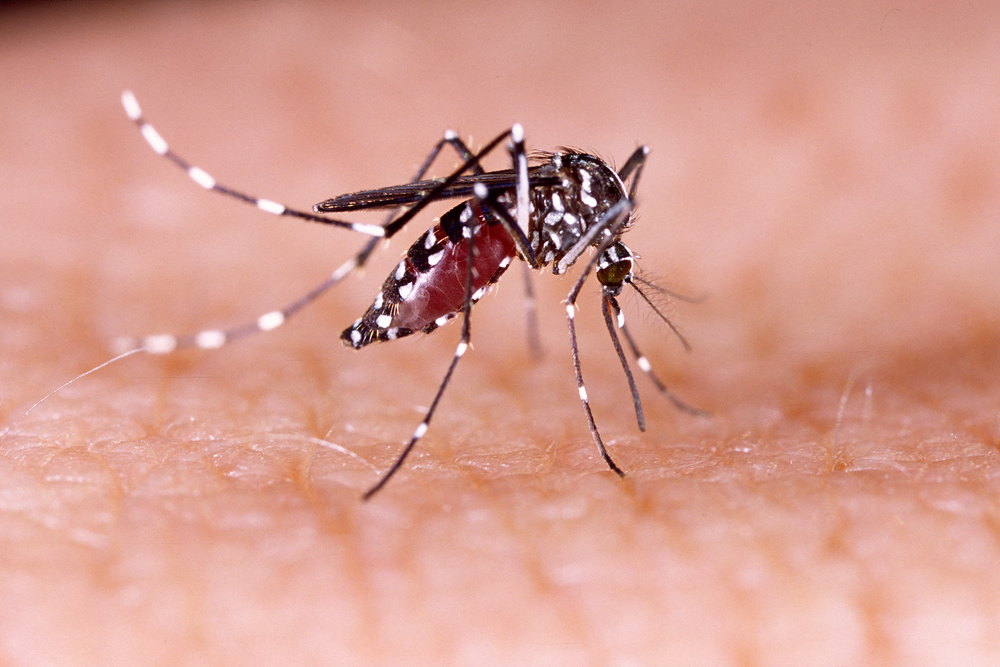
What is chikungunya?
Chikungunya is a viral disease transmitted by the Aedes aegypti mosquito. Some cases may be asymptomatic, while others can cause a fever above 38.5°C (101.3°F) and intense joint pain in the hands and feet, along with headaches, muscle pain, and red skin rashes.
The main public health impact lies in the sequelae left by the disease – severe joint pain can become chronic and last for years. Also, cases of death associated with the disease have been recorded in Brazil in the last few years.
According to the World Health Organization (WHO), the chikungunya virus has been identified in 110 countries in Asia, Africa, Europe, and the Americas. In Brazil, cases increased by more than 100% between 2021 and 2022.
There is currently no specific treatment for the infection, and the main form of prevention is vector control, similar to the dengue and zika viruses. This includes regularly emptying and cleaning containers with stagnant water, such as plant pots, buckets, tires and plastic bottles.
According to Moreira, while waiting for Anvisa’s response, the Butantan Institute has already started negotiations with the Ministry of Health to provide the vaccine free of charge in Brazil’s public health system (SUS).
“The Butantan Institute is already negotiating with the Ministry of Health to better understand the demands of the vaccine and the best implementation strategies in the country,” he said.
An important aspect of the Anvisa evaluation is that it will be conducted jointly with the European Medicines Agency (EMA), the regulatory agency of the European Union (EU). This is the first time that Anvisa and EMA will work together in the evaluation of a vaccine.
In November, the same vaccine was already approved for use in the United States by the Food and Drug Administration (FDA), the US regulatory agency.










